
Ongoing Monitoring
ongoing-monitoring.RmdAs with any model that is in production, it’s important to continuously monitor a scorecard model’s performance to ensure its robustness and ability to separate a “good” loan from a “bad” loan.
This vignette details how to perform the following monitoring exercises:
- Create Score Range Bins for Assessing Population Stability
- Calculate the Population Stability Index
- Calculate the Chi-Squared Goodness of Fit Test
- Calculate the Characteristic Analysis Index
- Compare Multiple Monitoring Time Horizon Windows
The vignette concludes with additional considerations for utilizing domain experts, monitoring variables not currently in the production model, and what to do when your monitoring processes indicate that your production model is weakening.
Similar to the {KAscore} package itself, the content herein is largely based upon the book Intelligent Credit Scoring: Building and Implementing Better Credit Risk Scorecards by Naeem Siddiqi (2nd Edition).
Terminology
Throughout this vignette, we will refer to the “development” sample and the “monitoring” sample.
The development sample is the data that was used to develop the scorecard model that is currently in production.
The monitoring sample is the new data that has been scored by the model since it was put into production.
Population Stability
The population stability index compares the sample distributions between the development data and the monitoring data across score ranges.
Said another way, this index compares the proportion of loans in the development data that scored (for example) between 200-210 with the proportion of loans in the monitoring sample that scored between 200-210; then compares the proportion of loans in the development data that scored (for example) between 211-220 with the proportion of loans in the monitoring sample that scored between 211-220; and so on. This index helps answer the question: “Does our recent applicant pool continue to look like the applicant pool that the model was trained on?”
There are multiple ways to specify the score ranges necessary to develop the population stability index, including:
- Set score ranges such that you end up with an equal number of
development sample cases in each bin; the
bin_quantile()function from {KAscore} can automatically compute equally sized range bins - Set uniform score ranges that are (for example) 20 points apart; i.e., (300, 320], (320, 340], etc; his approach can provide “cleaner” cutoff values within the ranges, but may result in some bins that have very few development sample cases
- Define custom score ranges that are perhaps the result of a hybrid approach between the two methodologies above (e.g., adjust the quantile bins slightly so that the range cutoffs to have “cleaner” breaks)
Developing Score Range Bins
Suppose we used the woe(), glm(), and
points() functions (as described in Build a Scorecard) to create the
following scorecard that is being used in production:
| Variable | Class | Points |
|---|---|---|
| industry | 110 | |
| industry | beef | 69 |
| industry | dairy | 73 |
| industry | fruit | 65 |
| industry | grain | 87 |
| industry | greenhouse | 61 |
| industry | nuts | 67 |
| industry | pork | 58 |
| industry | poultry | 97 |
| industry | sod | 71 |
| housing_status | own | 79 |
| housing_status | rent | 67 |
| collateral_type | building society savings agreement/ life insurance | 75 |
| collateral_type | car or other, not in attribute Savings account/bonds | 75 |
| collateral_type | real estate | 85 |
| collateral_type | unknown / no property | 63 |
Now suppose this card has been in production for a month, and 200 new loans have been scored using the model. We can view the first few loans in this monitoring sample in the table below:
| Loan ID | Industry | Housing Status | Collateral Type | Points Scored |
|---|---|---|---|---|
| 500001 | fruit | own | building society savings agreement/ life insurance | 219 |
| 500002 | dairy | rent | real estate | 225 |
| 500003 | grain | rent | car or other, not in attribute Savings account/bonds | 229 |
| 500004 | poultry | own | car or other, not in attribute Savings account/bonds | 251 |
| 500005 | grain | rent | building society savings agreement/ life insurance | 229 |
| 500006 | grain | rent | car or other, not in attribute Savings account/bonds | 229 |
From a monitoring perspective, we want to compute the population stability index to ensure that the data scored by the model in the last month aligns with the data used to develop the model.
First, we will need to assign points to our development sample (the number of points each applicant in the development sample would have scored if the model was in production).
| Loan ID | Industry | Housing Status | Collateral Type | Points Scored |
|---|---|---|---|---|
| 100100 | grain | own | real estate | 251 |
| 100101 | grain | own | real estate | 251 |
| 100102 | pork | own | real estate | 222 |
| 100103 | dairy | rent | building society savings agreement/ life insurance | 215 |
| 100104 | fruit | rent | unknown / no property | 195 |
| 100105 | pork | rent | unknown / no property | 188 |
Next, we have to create score range bins using the scores our
development sample data would have received. The
bin_quantile() function from {KAscore} may provide a good
starting point.
# Create six score range bins using the scores from the development sample
development_sample <- development_sample |>
dplyr::mutate(
bin = bin_quantile(
points,
n_bins = 6L,
min_value = min(points),
max_value = max(points),
)
)
# Show score range bins & proportion of the development sample in each of them
development_score_ranges <- development_sample |>
dplyr::count(bin) |>
dplyr::mutate(prop = n / sum(n))
development_score_ranges |>
dplyr::mutate(prop = scales::percent(prop)) |>
knitr::kable(
col.names = c(
"Score Range",
"# of Cases in Development Sample",
"% of Development Sample"
),
align = c("l", "r", "r"),
caption = "Score Range Bins (Using Development Sample)"
)| Score Range | # of Cases in Development Sample | % of Development Sample |
|---|---|---|
| [188,215] | 192 | 19.2% |
| (215,221] | 142 | 14.2% |
| (221,227] | 194 | 19.4% |
| (227,237] | 153 | 15.3% |
| (237,241] | 166 | 16.6% |
| (241,274] | 153 | 15.3% |
Note that even when performing quantile binning or manual binning, it may be difficult to achieve a perfectly equal proportion of the data across all score range bins, depending on your scorecard. By definition, scorecards have a finite number of possible of points that an applicant can receive, based upon the different combinations of the loan applicant attributes (i.e., the classes within each independent variable). For scorecards with fewer independent variables (and/or few classes within the independent variables), this can result in large proportions of the development sample data that have the exact same score. Because we are binning integers, this can result in bins that are not perfectly equal in size.
It’s important to ensure that you have enough data in each score bin. Each of the six score bins we created in the table above contains at least 10% of the development sample data. In a perfect world, we would have 16.7% of the data (100 divided by 6 score range bins) in each bin.
Now we can apply these score range bins to our monitoring sample to see how the distribution of data within each bin compares across the two samples.
# Capture the score range bins from the development sample as a vector that can be
# passed to `cut()`, so that we can apply the same bin breaks to the monitoring
# sample
points_cuts <- gregexpr(
pattern = "[0-9]+",
text = as.character(development_score_ranges$bin)
)
points_cuts <- regmatches(
x = as.character(development_score_ranges$bin),
m = points_cuts
) |>
unlist() |>
unique()
# Apply the score range bins to the monitoring sample
monitoring_sample <- monitoring_sample |>
dplyr::mutate(
bin = cut(
points,
breaks = points_cuts,
right = TRUE,
include.lowest = TRUE
)
)
monitoring_score_ranges <- monitoring_sample |>
dplyr::count(bin) |>
dplyr::mutate(prop = n / sum(n))
# Create a summarized table, grouped by score range bin, that can be compared to the
# development sample
monitoring_score_ranges |>
dplyr::mutate(prop = scales::percent(prop)) |>
knitr::kable(
col.names = c(
"Score Range Bin",
"# of Cases in Monitoring Sample",
"% of Monitoring Sample"
),
align = c("l", "r", "r"),
caption = "Score Range Bins (Applied to Monitoring Sample)"
)| Score Range Bin | # of Cases in Monitoring Sample | % of Monitoring Sample |
|---|---|---|
| [188,215] | 29 | 14.5% |
| (215,221] | 24 | 12.0% |
| (221,227] | 30 | 15.0% |
| (227,237] | 35 | 17.5% |
| (237,241] | 29 | 14.5% |
| (241,274] | 53 | 26.5% |
Now we can compare the distribution of the data in each score range bin between the development and monitoring samples:
# Join the monitoring summary table to the development summary
comparison_table <- development_score_ranges |>
dplyr::left_join(
monitoring_score_ranges,
by = "bin",
suffix = c("_development", "_monitoring")
)
# Show the comparison table
comparison_table |>
dplyr::select(-(dplyr::starts_with("n_"))) |>
dplyr::mutate(
dplyr::across(
.cols = -bin,
.fns = scales::percent
)
) |>
knitr::kable(
col.names = c(
"Score Range Bin",
"% of Development Sample",
"% of Monitoring Sample"
),
align = c("l", "r", "r"),
caption = "Comparison of Score Range Distributions"
)| Score Range Bin | % of Development Sample | % of Monitoring Sample |
|---|---|---|
| [188,215] | 19.2% | 14.5% |
| (215,221] | 14.2% | 12.0% |
| (221,227] | 19.4% | 15.0% |
| (227,237] | 15.3% | 17.5% |
| (237,241] | 16.6% | 14.5% |
| (241,274] | 15.3% | 26.5% |
You may prefer to visualize this same comparison data in a chart, as opposed to a table:
comparison_table |>
dplyr::select(-(dplyr::starts_with("n_"))) |>
# Convert data to long format
tidyr::pivot_longer(
cols = -bin,
names_to = "prop_sample",
values_to = "value"
) |>
# Improve labels
dplyr::mutate(
prop_sample = dplyr::case_when(
prop_sample == "prop_development" ~ "Development",
prop_sample == "prop_monitoring" ~ "Monitoring",
)
) |>
ggplot2::ggplot() +
ggplot2::geom_bar(
mapping = ggplot2::aes(
x = prop_sample,
y = value,
fill = bin
),
stat = "identity",
color = "black",
position = ggplot2::position_fill(reverse = TRUE)
) +
ggplot2::scale_y_continuous(labels = scales::label_percent()) +
ggplot2::coord_flip() +
ggplot2::scale_fill_brewer(
name = "Score Range Bin",
palette = "RdYlGn",
direction = 1
) +
ggplot2::labs(
title = "Comparison of Score Range Distributions",
x = "Sample",
y = "Distribution of Sample"
) +
ggplot2::theme_bw()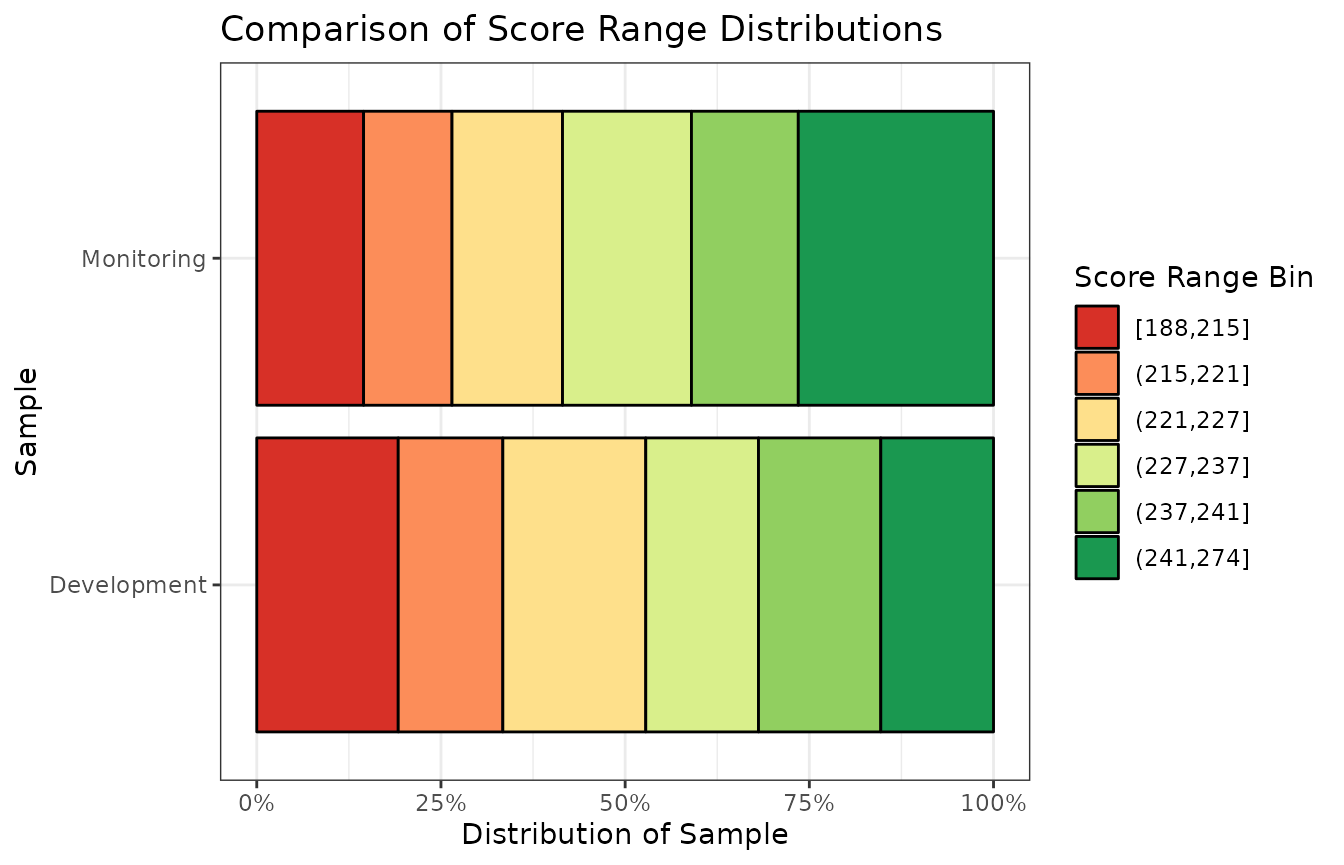
Population Stability Index
Once we have our development and monitoring data summarized by score ranges, we can compute the Population Stability Index via the formula below:
\[ \sum \Bigg[ \Big( \text{% Monitoring}_{b} - \text{% Development}_{b} \Big) * ln \Bigg( \frac{ \text{% Monitoring}_{b}}{ \text{% Development}_{b}} \Bigg) \Bigg] \] for each unique score range bin \(b\).
The index can be interpreted as follows:
- A value less than
0.1shows no significant change - A value between
0.1and0.25denotes a small change that needs to be investigated - A value greater than
0.25points to a significant shift in the applicant population
We can use the psi() function from {KAscore} to compute
the population stability index value for the monitoring sample:
# Compute the Population Stability Index
population_stability_index <- psi(
x = development_sample$bin,
y = monitoring_sample$bin
)The population stability index value is 0.1, which indicates that
there is a small change that needs to be investigated. In the comparison
table, we see that the percentage of monitoring cases in the
(241, 274] score range bin is higher than in the
development sample. We can also see that the percentage of
monitoring cases in the [188, 215] score range bin
is lower than in the development sample. This indicates that
our applicant pool is actually scoring better (i.e., higher) than the
applicant pool used to develop the model.
Chi Squared Goodness of Fit Test
We can also perform a Chi-Squared Goodness of Fit Test as another way to determine if the two samples’ score range distributions are statistically different. The null hypothesis of this test is that the two samples being compared were drawn from the same population (i.e., the applicant pool within the monitoring sample behaves similarly to the applicant pool within the development sample). A small p-value (e.g., less than 0.05) would cause us to reject the null hypothesis, and lead us to believe that the monitoring sample is showing a shift in the applicant pool being scored by our model.
# Compute the Chi-Squared test
chi_sq <- rbind(
comparison_table$n_development,
comparison_table$n_monitoring
) |>
as.table() |>
chisq.test()
# Show the test results
chi_sq
#>
#> Pearson's Chi-squared test
#>
#> data: as.table(rbind(comparison_table$n_development, comparison_table$n_monitoring))
#> X-squared = 17.457, df = 5, p-value = 0.00371We see that the p-value is 0.004. If we are using a significance threshold of 0.05, this would mean that the applicant pool within the monitoring sample behaves differently than the applicant pool that was used to develop the model. This result would imply that a change to the model should be explored (e.g., consider replacing and/or adding independent variables, re-training the model using data from a window closer to present, weight newer cases in the training data heavier than older cases).
Characteristic Analysis Index
Shifts in the distributions of the scores can only be caused by the independent variables in the scorecard. While the population stability index details shifts in the population at the level of the overall model, the characteristic analysis index similarly details shifts in the population within each independent variable.
The characteristic analysis index is computed as follows:
\[ \sum \Big( \text{% Monitoring}_{c} - \text{% Development}_{c} \Big) * \text{Points}_{c} \]
for each unique level \(c\) in the independent variable being analyzed.
Let’s take a look at an example of a characteristic analysis for the
Industry independent variable, using the
cai() function from {KAscore}.
# Select rows of interest from our card
card_industry_points <- card |>
dplyr::filter(variable == "industry") |>
dplyr::select(-variable)
# Perform the analysis
characteristic_analysis <- cai(
data = card_industry_points,
x = development_sample$industry,
y = monitoring_sample$industry,
verbose = FALSE
)
# Show the characteristic analysis table
characteristic_analysis$classes |>
dplyr::mutate(
dplyr::across(
.cols = dplyr::starts_with("prop_"),
.fns = scales::percent
)
) |>
knitr::kable(
col.names = c(
"Industry",
"% of Development Sample",
"% of Monitoring Sample",
"Points",
"CAI"
),
align = c("l", "r", "r", "r", "r"),
caption = "Characteristic Analysis Index - Industry"
)| Industry | % of Development Sample | % of Monitoring Sample | Points | CAI |
|---|---|---|---|---|
| 0.9% | 0.5% | 110 | -0.440 | |
| beef | 9.7% | 10.5% | 69 | 0.552 |
| dairy | 18.1% | 15.0% | 73 | -2.263 |
| fruit | 23.4% | 19.0% | 65 | -2.860 |
| grain | 28.0% | 25.0% | 87 | -2.610 |
| greenhouse | 1.2% | 0.5% | 61 | -0.427 |
| nuts | 2.2% | 1.5% | 67 | -0.469 |
| pork | 5.0% | 4.0% | 58 | -0.580 |
| poultry | 10.3% | 22.5% | 97 | 11.834 |
| sod | 1.2% | 1.5% | 71 | 0.213 |
Looking at the individual values in the table above, we can see that the new loan applicant pool (the monitoring sample) are shifting towards more poultry loans, and moving slightly away from almost all of the other industries.
The total characteristic analysis index value for
Industry, which can be accessed through
characteristic_analysis$net, is equal to 3. In other words,
within the industry independent variable, new loan applicants
(in the monitoring sample) are scoring 3 points higher than the
applicant pool in the development sample. This may lead us to
investigate what led to the increase in poultry loan applications last
month (marketing campaign, seasonality, etc.).
To complete the characteristic analysis index for our model, we could repeat this exercise for the other independent variables in our model (Housing Status and Collateral Type).
Comparing Multiple Monitoring Windows
The analysis performed so far indicates that there are differences in the score distributions between development sample and applicants who were scored by the model last month (i.e., the monitoring sample). As you collect more data for monitoring purposes, it can be useful to create multiple monitoring “windows” for analysis. For example, creating monitoring samples of data from the past month, three months prior, six months prior, etc., can help detect any emerging trends or confirm that any deviations in one particular month do not represent longer-term trends.
Let’s complement our analysis with data corresponding to applicants
from three (three_months_ago_sample) and six
(six_months_ago_sample) months ago.
We can use the following code to compute the score range distributions:
# Simulate a monitoring sample from 6 months ago
six_months_ago_sample <- loans |>
dplyr::select(industry, housing_status, collateral_type) |>
dplyr::sample_n(size = 250) |>
dplyr::mutate(loan_id = dplyr::row_number() + 200000)
# Simulate a monitoring sample from 3 months ago
three_months_ago_sample <- loans |>
dplyr::select(industry, housing_status, collateral_type) |>
dplyr::sample_n(size = 300) |>
dplyr::mutate(loan_id = dplyr::row_number() + 400000)
# Add the score range bins and points to the monitoring sample from 6 months ago
six_months_ago_score_ranges <- six_months_ago_sample |>
tidyr::pivot_longer(
cols = -loan_id,
names_to = "variable",
values_to = "class"
) |>
dplyr::left_join(card, by = c("variable", "class")) |>
dplyr::summarise(
points = sum(points),
.by = loan_id
) |>
dplyr::mutate(
bin = cut(
points,
breaks = points_cuts,
right = TRUE,
include.lowest = TRUE
)
) |>
dplyr::count(bin) |>
dplyr::mutate(prop = n / sum(n)) |>
dplyr::select(-n)
# Add the score range bins and points to the monitoring sample from 6 months ago
three_months_ago_score_ranges <- three_months_ago_sample |>
tidyr::pivot_longer(
cols = -loan_id,
names_to = "variable",
values_to = "class"
) |>
dplyr::left_join(card, by = c("variable", "class")) |>
dplyr::summarise(
points = sum(points),
.by = loan_id
) |>
dplyr::mutate(
bin = cut(
points,
breaks = points_cuts,
right = TRUE,
include.lowest = TRUE
)
) |>
dplyr::count(bin) |>
dplyr::mutate(prop = n / sum(n)) |>
dplyr::select(-n)We can compare the score distribution of development sample and the monitoring samples for several historical periods with the following code:
dplyr::bind_rows(
# Development sample
development_score_ranges |>
dplyr::select(-n) |>
dplyr::mutate(sample = "Development"),
# Last month's monitoring sample
monitoring_score_ranges |>
dplyr::select(-n) |>
dplyr::mutate(sample = "Monitoring (Last Month)"),
# Monitoring sample from 3 months ago
three_months_ago_score_ranges |>
dplyr::mutate(sample = "Monitoring (3 Months Ago)"),
# Monitoring sample from 6 months ago
six_months_ago_score_ranges |>
dplyr::mutate(sample = "Monitoring (6 Months Ago)")
) |>
ggplot2::ggplot(
mapping = ggplot2::aes(
x = bin,
y = prop,
group = sample,
color = sample
)
) +
ggplot2::geom_line() +
ggplot2::geom_point() +
ggplot2::scale_y_continuous(labels = scales::label_percent()) +
ggplot2::labs(
title = "Popluation Stability Chart",
subtitle = "Across multiple monitoring windows",
x = "Score Range",
y = "Distribution of Sample",
color = NULL # Remove legend title
) +
ggplot2::theme_bw()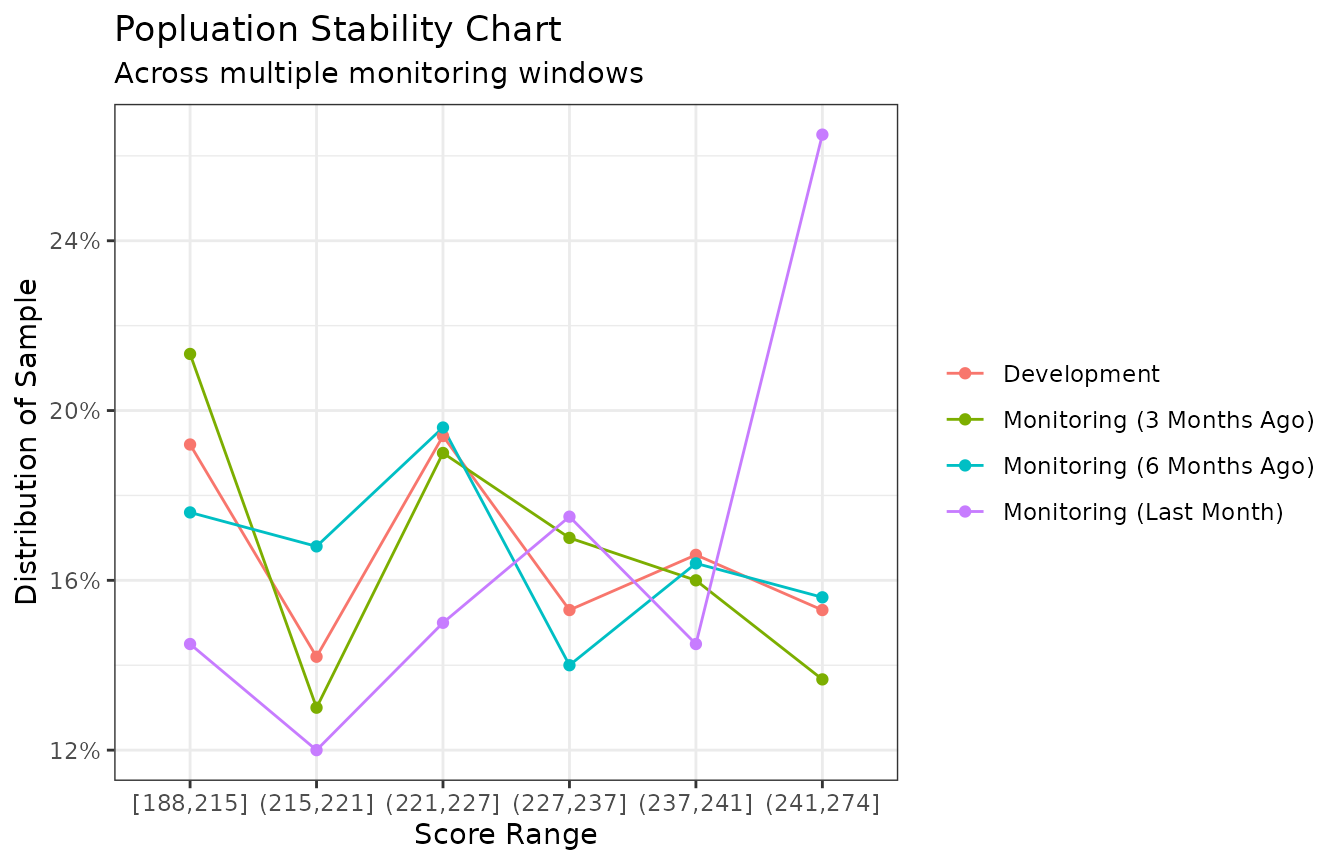
We see that applicants from last month show a deviation from the loan applicant pool used during development, especially in the highest score range bin, (241, 274]. However, the next most recent monitoring sample (from 3 months ago) shows a distribution much closer to the development sample across all score range bins. This tells us that the differences between last month’s monitoring sample and the development sample may be due to random noise, as opposed to any trend taking place that would indicate a shifting population.
If, instead, distributions for the monitoring samples from three and six months ago also showed deviations similar to the monitoring sample from last month, we would conclude that the trend started more than six months ago and we may want to consider re-training the scorecard.
Additional Considerations
The population stability index, Chi-Squared test, and characteristic analysis index should all be taken into consideration alongside subject matter expertise. Understanding economic trends that affect your loan applicant pool can provide the context necessary to make decisions on whether or not to alter your scorecard model. It’s important to consider what information outside of your scorecard (i.e., information that is not represented in the independent variables of your model) may be having an impact on its performance since it was developed.
While the population stability index, Chi-Squared test, and
characteristic analysis index statistics focus on the current schema of
the model in production, it is good practice to continue to monitor
other attributes that were considered to be independent variables during
development, but were not incorporated in the production model due to
weak signal. As you continue to gather more performance data over time,
the information value statistic (via the iv() function from
the {KAscore} package) for each of these unused attributes should be
re-computed periodically to assess if they should be incorporated into
the model to boost predictive accuracy. Conversely, the information
value for each independent variable in the production model should also
be periodically re-computed to ensure it is not decreasing the model’s
predictive accuracy.
In the event that a significant shift in the loan applicant population has taken place since the model was developed, the most important first step is to work with domain experts to try to identify or hypothesize what caused the shift. If possible, engineer one or more features (i.e., potential independent variables) using this hypothesis and relevant available data, develop a “competitor” scorecard model using these new independent variable(s) (and removing any that are hindering the production model’s ability to recognize the shift taking place), and compare the competitor model’s accuracy to the production model’s accuracy using a held-out test sample that encompasses a more recent population than what was used to train the current model in production.